
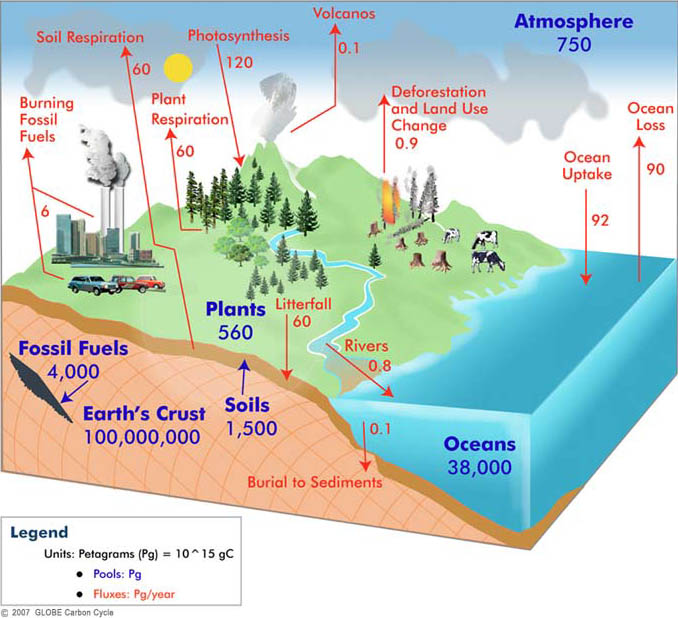
^ Has an important use in radiodating (see carbon dating).^ Ratio of 12C to 13C used to measure biological productivity in ancient times and differing types of photosynthesis.^ The unified atomic mass unit is defined as 1/12 of the mass of an unbound atom of carbon-12 in its ground state.^ a b c d e Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.^ Used for labeling molecules in PET scans.^ Immediately decays by proton emission to 4.^ Subsequently decays by double proton emission to 4.^ # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).^ ( ) spin value – Indicates spin with weak assignment arguments.^ Bold symbol as daughter – Daughter product is stable.
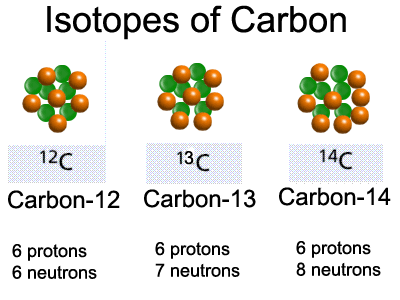

Carbon ( 6C) has 15 known isotopes, from 8Īre stable.


 0 kommentar(er)
0 kommentar(er)
